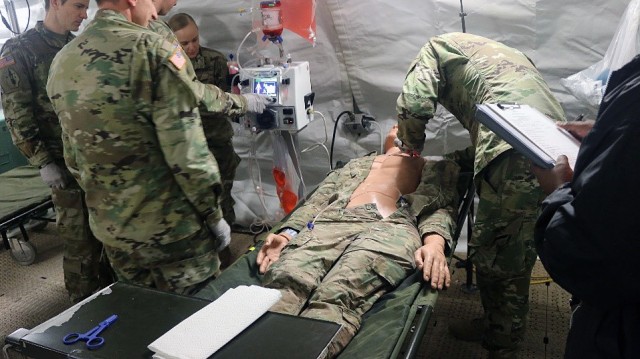
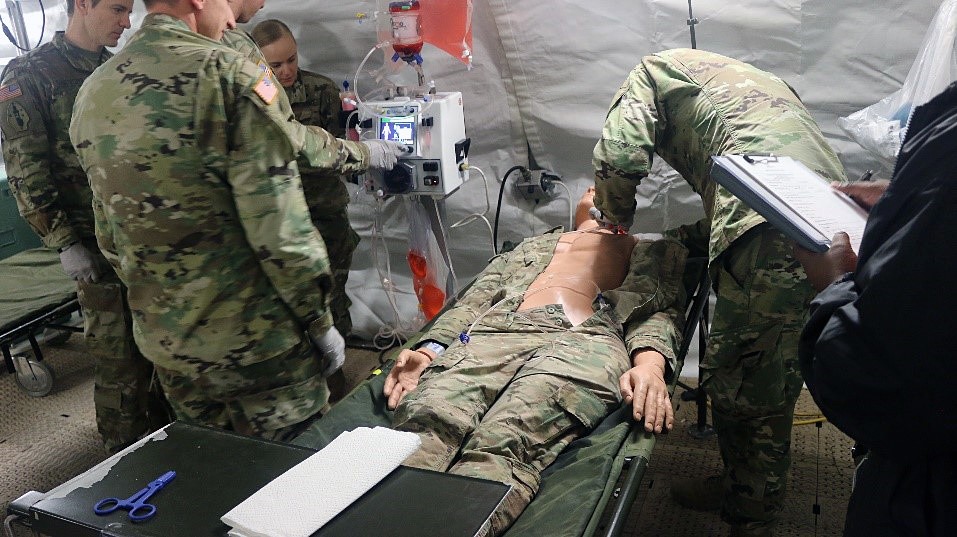
JOINT BASE SAN ANTONIO, TX - The U.S. Army Medical Department Board, U.S. Army Medical Center of Excellence, performed a test at Camp Bullis, TX that could dramatically improve battlefield survivability. The team conducted a field test of the Extracorporeal Life Support (ECLS) from 18-20 February at the Deployable Medical Systems Equipment for Training (DMSET) site. Hospitals have used ECLS, also called extracorporeal membrane oxygenation (ECMO), to treat patients who cannot oxygenate their blood through breathing. Conducting the test in a field environment marks a departure from traditional brick-and-mortar facilities where ECLS systems are normally used. During the test event, both Army medical officers and enlisted personnel participated in scenarios. Army civilians and Soldiers conducted the tests.
ECLS involves the artificial support of lung and/or heart function using a machine that oxygenates a patient's blood outside the body and returns it using a pump, thus allowing the heart and lungs to rest. When a patient is connected to an ECLS circuit, blood flows through a series of tubes to an artificial lung that adds oxygen and takes out carbon dioxide. The blood is then returned to the body through the ECLS circuit using a specialized pump. The device that was tested also provides continuous renal replacement therapy for treatment of acute kidney injuries.
Army healthcare professionals assigned to Joint Base San Antonio and Joint Base Lewis-McChord participated in the test event. The test was requested by the Warfighter Expeditionary Medicine and Treatment (Warfighter EMT) project management office of the U.S. Army Medical Materiel Development Activity (USAMMDA). The Warfighter EMT is working to develop medical capabilities for the combat environment that are normally used to treat wounds in hospital facilities.
Expanding the use of these systems to deployable field care would be a significate advance in expanding prolong field care. "Organizations like the USAMEDDBD and USAMMDA are working diligently to ensure that Army Medicine has the capabilities required to sustain survival rates during the future fight," said CW3 Goldie Cooper with the AMEDD Board. "Conducting test and evaluation of advanced medical capabilities like ECLS is a step towards improving prolonged field care methods and saving lives on the battlefield."
As stated in a publication by the U.S .National Library of Medicine National Institutes of Health the first clinical cases of ECLS for heart and lung failure were in the 1970s with success treating neonatal patients. In the early 1980s, two centers conducted randomized trials in neonates, which demonstrated much higher survival with ECLS, and the term extracorporeal membrane oxygenation (ECMO) was coined. By 1986, there were 18 ECMO neonatal centers and data on 700 cases. In the 1990s, these centers expanded indications to older children with lung and heart failure. ECMO management for post-operative cardiac failure became standard in major pediatric heart centers. Currently, ECMO is used for severe heart and lung failure in all ages.
The results of the entire test event will be provided to the USAMMDA Warfighter EMT Product manager in an operational test report to help determine feasibility for deployment with operational units.
Related Links:
U.S. Army Medical Department Board








Social Sharing