FORT DETRICK, Md. – A process developed by the Medical Research and Development Command’s Medical Technology Transfer Office to help inventors ensure their biomedical technologies are ready for commercialization has been recognized by the Federal Laboratory Consortium for Technology with a prestigious 2024 Technology Transfer Innovation Award.
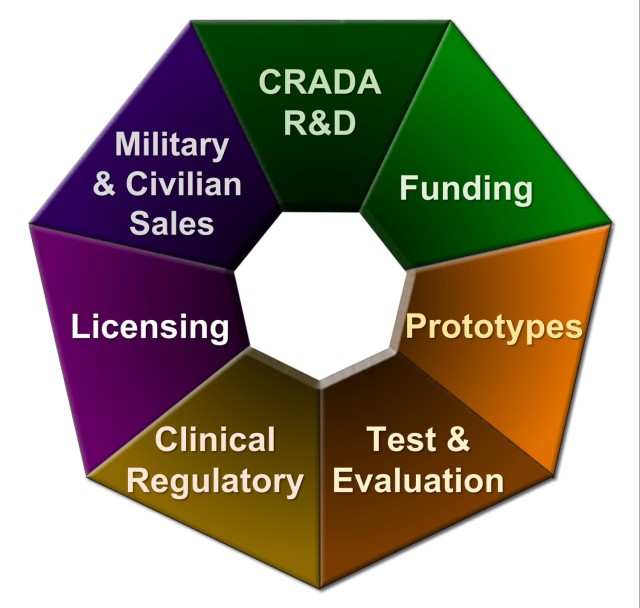
MTT’s Assistive Technology Transfer process, or AT2, connects inventors with expert assistance at each stage of their inventions’ development, particularly during the critical early stages of prototyping and field testing. AT2 helps them identify and gain access to funding sources, meet experts who can answer questions about regulatory and licensing requirements, obtain resources for developing and testing prototypes and assist with military and civilian sales and licensing, among other support. This helps ensure that the new technologies will be mature and ready for manufacture, increasing the likelihood that they will be licensed.
“This award recognizes our excellence in fulfilling our responsibility to get inventions licensed, patented and out into the commercial sector” says Dr. Paul Michaels, MTT’s director. “Since implementing the AT2 process, we’ve seen substantial growth in the number of inventions being licensed.”
To date, MTT has successfully used the AT2 process to help more than 20 biomedical technologies achieve commercial and military sales, which has generated over $26 million in licensing revenue over the last five years. MTT has used that revenue to reward inventors and has also invested it back into the AT2 program to help move other new inventions through the development cycle faster and more effectively – creating what MTT calls a feedback loop of success.
MTT developed the AT2 process to help inventors of new biomedical technologies navigate the complex process of obtaining funding to develop their products, collaborate with Defense Health Agency labs and medical treatment facilities and sell their product to the DOD. By helping ensure that inventors and their commercial partners complete each step at the right time, in the right order, the AT2 process creates what MTT calls a bridge of hope that enables them to cross what many in the field refer to as technology transfer’s valley of death.
Among the innovative prototypes that the AT2 process is currently helping guide across the valley are:
• An improved surgical gown that has specially designed pockets that help doctors maintain sterility in a wide range of challenging environments.
• An ocular chemical injury treatment device that provides deep cleaning of hard-to-reach corners of the eye and restores the eye’s pH balance.
• A plastic bite block that prevents facial scarring for intubated burn patients.
• An inflatable integrated litter and shelter for conducting casualty evacuations in the Arctic and other extreme environments.
“We developed the AT2 process because we needed to field products that still had a lot of risk associated with them and we don’t want people to have to beg, borrow or steal in order to accomplish their goals,” says Barry Datlof, MRDC’s chief of business development and commercialization. “We have the resources to help inventors de-risk their inventions to make sure they are ready to be brought to market.”
While many of the inventors that MRDC’s MTT works with are associated with one of the command’s laboratories, such as the Walter Reed Army Institute of Research or the Medical Research Institute of Infectious Diseases, the team is willing to work with government-related inventors who have a good idea but may lack institutional support.
For example, the inflatable litter and shelter designed for arctic casualty evacuations was invented by a member of the Alaska National Guard.
“He’s not an engineer or a scientist,” explains Blake Sajonia, a technology transfer professional with MTT. “He showed it to his chain of command and they liked it – and then they asked him how soon he could get one built! He ended up finding us and we agreed to take it on. We serve as a catch-all for inventions that don’t have a home elsewhere.”
Ron Marchessault, a fellow MTT technology transfer professional, says that one of the advantages of AT2 is that when the process is completed, potential licensors receive not just the intellectual property of the invention, but also extensive test data, prototypes, CAD files and other supporting documentation that demonstrates the readiness of the technology for mass manufacture.
“That way, a commercialization partner can look at the IP and the data package that goes with it and they will have the full picture of the invention’s potential when deciding whether they want to pursue it,” says Marchessault.
Datlof says that his team would like to see the AT2 process be adopted as a government-wide standard for technology transfer.
“Many small technology transfer offices don't have the cash flow to be able to afford some of the things that we have been fortunate enough to afford,” Datlof says. “We would love to promulgate assistive technology transfer throughout DOD.”
FLC is a nationwide network of over 300 federal laboratories, agencies and research centers. Its mission is to encourage commercialization and to share best practices and opportunities for bringing federally developed technologies to the marketplace. The purpose of the Technology Transfer Innovation Award is to recognize successful implementations of “innovative or unconventional approaches, such as in-house competitions, to significantly increase the lab’s technology transfer partnerships and results.”
MTT’s winning team is in distinguished company at this year’s FLC National Meeting Awards; programs from the U.S. Departments of Energy, Health and Human Services and Veterans Affairs also won awards in the technology transfer category. Altogether, FLC is honoring 32 award winners from eight federal agencies this year. The award will be presented at a ceremony and banquet during the FLC’s annual national meeting April 9-11, 2024, at the Sheraton Dallas Hotel in Dallas, Texas.
For more information about MRDC’s Medical Technology Transfer office, visit: https://technologytransfer.health.mil.








Social Sharing