RESEARCH TRIANGLE PARK, N.C. -- A new U.S. Army-funded discovery could improve energy efficiency of technologies such as solar panels and fuel cells.
The research, funded by the Army Research Office, an element of the U.S. Army Combat Capabilities Development Command’s Army Research Laboratory, developed a novel technique to directly measure for the first time, energy distributions of hot electrons.
Hot electrons feature much larger energies than normal that can be generated in nanostructures.
Published in the journal Science, the research, conducted by a team of scientists from the University of Michigan and Purdue University, was part of the Department of Defense's Multidisciplinary University Research Initiatives program, supported by ARO.
“This multidisciplinary basic research effort sheds light on a unique way to measure the energy of charge carriers,” said Dr. Chakrapani Varanasi, an ARO program manager, who supported this study. “These results are expected to play a crucial role in developing future applications in energy conversion, photocatalysis and photodetectors, for instance, that are of great interest to the Department of Defense.”
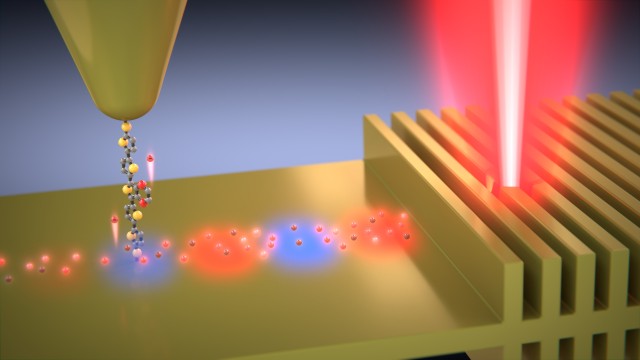
The team demonstrated how a technique using a scanning tunneling microscope integrated with lasers and other optical components reveals the energy distribution of hot electrons.
“For example, if you wanted to employ light to split water into hydrogen and oxygen, you can use hot charge carriers because electrons that are more energetic can more readily participate in the reaction and drive the reaction faster,” said Dr. Edgar Meyhofer, a professor of mechanical engineering at University of Michigan, who co-led the research along with professors Pramod Sangi-Reddy and Vladimir Shalaev.
It’s one possible use for hot carriers in energy conversion and storage applications, Meyhofer said.
Hot electrons are typically generated by shining a certain frequency of light on a carefully engineered nanostructure made of metals such as gold or silver, exciting so-called surface plasmons. These plasmons are believed to eventually lose some of their energy to electrons, making them hot. While hot electrons can have temperatures as high as 2,000 degrees Fahrenheit, it’s their high energy – rather than the material temperature – that makes them useful for energy technologies.
“Measuring energy distribution means quantifying how many electrons are available at a certain amount of energy,” said Harsha Reddy, a doctoral candidate in Purdue’s School of Electrical and Computer Engineering and co-lead author on this paper. “That crucial piece of information was lacking for expanding the use of hot electrons.”
The team created the hot electrons by shining laser light onto a gold film just 13 nanometers thick, or hundred or so gold atoms thick, with tiny ridges spaced so that they would resonate with the incident laser light and generate the surface plasmon waves. Then they measured the energies of the charge carriers by employing carefully chosen molecules.
These molecules, some of which were synthesized by collaborators at the University of Liverpool, allow only charge carriers with certain energies to pass. By filtering the charge carriers siphoned off the nanostructure, the researchers figured out the energy distribution of the charge carriers.
“Electrons can be thought of as cars running at different speeds on a highway,” said Dr. Kun Wang, a postdoctoral fellow at the University of Michigan. “The molecule acts like an operator—it only allows cars travelling at a certain speed to pass through.”
Wang spent more than 18 months working with Harsha Reddy on how to make this idea work. Once they had developed a successful method, Wang and Reddy repeated the experiments with a second gold structure, this one about 6 nanometers thick. The results showed that light generates hot charge carriers more efficiently on a thinner metal film, confirming theoretical predictions.
“These advances were achieved by insightfully combining nanotechnology tools that were developed at the University of Michigan and nanophotonic expertise from our collaborators at Purdue University, which was only possible due to a Multi[disciplinary] University Research Initiative effort supported by ARO,” Sangi-Reddy said.
With the method now demonstrated, the team believes that others can also use it to explore and optimize nanostructures.
The Department of Energy and the Office of Naval Research provided additional funding for the research. Seed funding from the U-M Department of Mechanical Engineering supported complementary calculations.
CCDC Army Research Laboratory is an element of the U.S. Army Combat Capabilities Development Command. As the Army's corporate research laboratory, ARL discovers, innovates and transitions science and technology to ensure dominant strategic land power. Through collaboration across the command’s core technical competencies, CCDC leads in the discovery, development and delivery of the technology-based capabilities required to make Soldiers more lethal to win the nation’s wars and come home safely. CCDC is a major subordinate command of the U.S. Army Futures Command.



Social Sharing