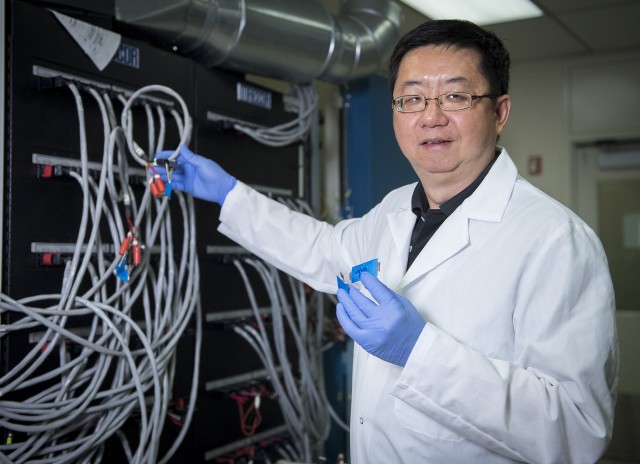
ADELPHI, Md. -- A team of scientists from the U.S. Army Research Laboratory, the University of Maryland and the Johns Hopkins University Applied Physics Laboratory have demonstrated a new type of flexible lithium-ion battery that not only poses no safety hazards, but also can continue to operate even under severe mechanical abuse.
Current Li-ion batteries used by Soldiers are not flexible and offer limited safety. In battle, these batteries will experience harsh abuses, especially when a Soldier has to drop and tumble on the ground when making tactical maneuvers. The state-of-the-art Li-ion batteries can't withstand repeated abuse, and once mutilated, will not only cease to work but causes hazards like fire, toxic fumes, unwanted thermal signals, and explosion.
Li-ion batteries have become the energy storage source of choice for multiple applications, ranging from consumer electronics, to military and aerospace systems, due to their excellent energy and power performance. Despite these benefits, potential safety hazards associated with the organic electrolytes that are used in Li-ion battery cells remain an ongoing concern. These electrolytes are highly flammable, toxic, and moisture sensitive, limiting the form factors in which a Li-ion battery can be manufactured.
Getting to a safer aqueous battery will eliminate hazardous concerns, said Dr. Kang Xu, Electrochemistry team leader and fellow at ARL. Tolerance against abuse, he added, makes it possible to design the Li-ion batteries to match shapes of Soldier body-worn gear without worrying about mishaps.
The work builds upon a novel aqueous electrolyte termed as "water-in-salt" developed in 2015 by ARL's Xu and UMD's Dr. Chunsheng Wang, professor of Chemical and Biomolecular Engineering. This highly concentrated water-based electrolyte can address the key issue associated with the use of water in Li-ion batteries, which is the low electrochemical stability window of roughly 1.2 V. By expanding this window to 3 V, which is more durable against abuse, the water-in-salt enables much higher energy density aqueous Li-ion batteries. Earlier this month, ARL and UMD published a paper in Joule that emphasized breaking into the 4 V realm, which carries higher energy and still makes the LIB safe but cannot work underwater.
By collaborating with JHU/APL, the team is starting to transition this technology into novel battery architectures and demonstrate its practical true potential, Wang said.
In this new research, the team is embedding the water-in-salt electrolyte in a polyvinyl alcohol polymer matrix, forming a gel polymer electrolyte. This GPE is even more stable than the liquid counterpart, and enables integration into a flexible battery configuration.
"What limits the form factor of current Li-ion batteries is the flammable organic electrolytes," said Dr. Kostas Gerasopoulos, senior research scientist and principal investigator at JHU/APL. "To ensure safety, you need sufficient packaging and protective measures. When the water-in-salt electrolyte was introduced, I thought that making a stable polymer version would radically change the way that Li-ion batteries are made and used."
The new polymer electrolyte does not act merely as a host for the liquid water-in-salt, rather it is a new type of aqueous solid electrolyte. The hydroxyl groups of the PVA participate in the solvation of the lithium salts and enable higher solubility, further widening the electrochemical stability window by 0.2 V and increasing the cycle stability and coulombic efficiency.
"By expanding the window of the electrolyte and improving its stability, we are also expanding the list of available materials that can be used to make working cells with long cycle life," Xu said.
The team's flexible battery uses LiVPO4F as the single active material in both the anode and cathode, forming a symmetric Li-ion battery. "LiVPO4F is not a new material. It is well established as a Li-ion battery cathode. What makes it attractive for us is that it has two distinct voltage plateaus at 2.0 V and 4.46 V (vs. Li+/Li) in water-in-salt electrolyte, both inside the stability window of the water-in-salt GPE (WISGPE). As a result, we can make a stable 2.4 V cell with just one material", said Dr. Chongyin Yang, an assistant research scientist in the University of Maryland.
The team operated the flexible Li-ion battery in open air with minimal packaging, using only some Kapton tape to keep the flexible substrate in place. In their demonstration, the battery powered a significant motor load without any safety concerns. To demonstrate the full safety potential, the team attempted further tests that are not possible with today's Li-ion batteries by cutting in the air or cut while immersing the battery in sea water when the battery is operation. Impressively, not only do these abuse tests cause no catastrophic failure, but the battery maintains its performance and continues to power the load even when completely exposed to air and water. The extraordinary safety at abuse condition for water-in-salt GPE LiVPO4F/LiVPO4F cell comes from the water in GPE strongly bond to the salt and WISGPE being slightly hydrophobic.
"We wanted to show the real implications of this technology in practical applications," Gerasopoulos said. "Particularly for our military, with our warfighters exposed to extreme conditions and environments during their missions, the capability to maintain both safety and performance is unprecedented."
"By making the batteries flexible and lighter compared to the devices currently used in the field, you can significantly decrease the burden to the warfighter", Xu said.
The current generation of flexible batteries shows huge potential, but they are still in the prototype phase. The team hopes that with the proper support, they can soon transition the technology to the warfighters. "We want to increase the robustness of the GPE and the energy density of the batteries even further. This work though proves the concept that we can build safe Li-ion batteries that can survive mechanical abuse", Gerasopoulos said.
"We are currently working on several key innovations both in the materials and manufacturing. We are interacting closely with the DoD community and we are very encouraged by the feedback we are receiving. We are not that far away from testing in the field. Sky is the limit for this technology", said Dr. Jeffrey P. Maranchi, Program Manager at JHU/APL.
Journal Reference:
Chongyin Yang, Xiao Ji, Xiulin Fan, Tao Gao, Liumin Suo, Fei Wang, Wei Sun, Ji Chen, Long Chen, Fudong Han, Ling Miao, Kang Xu, Konstantinos Gerasopoulos, Chunsheng Wang. Flexible Aqueous Li-Ion Battery with High Energy and Power Densities. Advanced Materials, 2017; DOI: 10.1002/adma.201701972
-----
The U.S. Army Research Laboratory, currently celebrating 25 years of excellence in Army science and technology, is part of the U.S. Army Research, Development and Engineering Command, which has the mission to provide innovative research, development and engineering to produce capabilities that provide decisive overmatch to the Army against the complexities of the current and future operating environments in support of the joint warfighter and the nation. RDECOM is a major subordinate command of the U.S. Army Materiel Command.

Social Sharing