JOINT BASE SAN ANTONIO-FORT SAM HOUSTON, Texas - The Chemical and Biological Defense Auto-Injector Device, or CBD auto-injector, is something no Soldier ever wants to use on the battlefield. The effects of chemical and biological weapons are horrific. Nerve agents are the most toxic of the known chemical agents. They are hazards in both liquid and vapor states and can cause death within minutes after exposure. Nerve agents are the primary chemical warfare agent threat because of their high toxicity and effectiveness through multiple routes of entry. They are absorbed through the eyes, respiratory tract, and skin. Ensuring CBD auto-injectors are functional and usable are critical in savings lives. The CBD Auto-Injector Device is designed to be used in a Chemical, Biological, Radiological, and Nuclear, or CBRN, environment.
Recently the U.S. Army Medical Department Board, or USAMEDDBD, assigned to the U.S. Army Medical Center of Excellence, conducted a test of these devices at Camp Bullis, Texas. The Joint Program Executive Office for Chemical and Biological Defense, Medical Countermeasure Systems, or JPEO--CBD MCS, at Fort Detrick, Maryland, requested the AMEDD Board conduct a customer test of the CBD auto-injector device within the operational environment. The data and test findings collected by the AMEDD Board will be will be provided to JPEO--CBD MCS.
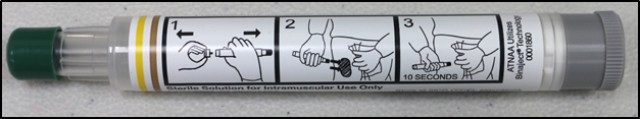
The CBD Auto-Injector Device is a replacement for the currently fielded auto-injector for treatment against nerve agent and insecticide poisoning, adjunctive treatment, and management of agent-induced seizures. The United States military adopted the auto-injector as the drug delivery device because of ease of use, packaging durability, and drug product stability under varying storage conditions.
Soldiers donned Mission Oriented Protective Posture, more commonly known as MOPP protective gear, and tested the CBD auto-injectors in a simulated battlefield exercise complete with smoke grenades. MOPA masks can limit visibility, so the injectors have clear, easy to understand pictorial instructions.
"A lot of these auto-injectors used to have wording, just written instructions," said Gary Cabigon, an Operational Tester with the AMEDD Board. "What we learned over the years is that pictures are easier to comprehend, especially in a stressful environment." Cabigon said that when under a CBRN attack vital seconds saved in dispensing the injector matter.
Sgt. First Class Elijah Williamson, an AMEDD Board Test Officer, talked about conducting the test in the field. "I think it's great having the training asset here at Fort Sam," said Williamson. We have the austere environment where we can create the simulation of the battlefield. We do so much on slides and videos that you want to get hands-on and create that realism out here at Camp Bullis. You can't get that in the classroom."
The CBD auto-injectors are designed to deliver an intramuscular injection with a 22-gauge needle with a pressure-activated coil spring mechanism that triggers the needle after removal of the safety cap. When activated, the needle protrudes through the needle end. The CBD auto-injector device is a replacement for the currently fielded auto-injector for treatment against nerve agent and insecticide poisoning, adjunctive treatment, and management of agent-induced seizures. The United States military adopted the auto-injector as the drug delivery device because of ease of use, packaging durability, and drug product stability under varying storage conditions.
To learn more about the Army Medical Department Board's mission, visit https://www.cs.amedd.army.mil/ameddbd.










Social Sharing