OTAs are charting a new path to make sure warfighters in the future fight get innovative care.
Just a few hours after a trio of late-night shootings one early August weekend in Denver, Humacyte Inc. CEO Jeffrey Lawson received an email from a local surgeon who tended to one of the victims.
"He just sent me a quick message," said Lawson, a trained surgeon who's based out of the biotechnology firm's headquarters in North Carolina. "He wanted to tell me how he used our product to repair circulation in the shooting victim's left arm."
That lifesaving product was a pioneering technology called the human acellular vessel (HAV), which is an off-the-shelf, bioengineered blood vessel currently being studied for the repair and reconstruction of the various types of vascular injuries sustained in both military and civilian conflicts-in the case of the victim in Denver, domestic trauma.
"In simpler terms, the HAV is a regenerative vascular implant that physically becomes part of the patient," said Humacyte Inc. co-founder Juliana Blum. "And so for us, having military support has really been a key piece of our puzzle."
Indeed, for DOD, that kind of capability-to limit blood loss, to restore blood flow to extremities-represents the future: the future of innovation, the future of medicine; in short, the future of a more resilient warfighter. Yet for years, the frustrating snags in DOD's plan to foster medical innovation were always the twin barbs of speed and collaboration. After all, what exactly is the simplest, most efficient way for an innovative company to dive into the complex world of military medical contracting with designs on creating, testing and finally delivering to the front lines a lifesaving, game-changing product?
Enter the other-transaction agreement.
INCREASED SPEED, FLEXIBILITY
In DOD, as everywhere, every second that ticks off the clock brings you one step further into the future and one step closer to your next obstacle. As the nation's military medical apparatus prepares for the future battlefield with a variety of concepts, tactics and products, it must also find a way to apply those tools quickly and efficiently, and at a pace likely exceeding those for previous efforts. For the Soldier entering the "future fight" on new and different battlefields-presumed to be thickly populated urban cityscapes where tools will need to be tougher, smaller and better than ever-nothing less will do.
In 2014, the U.S. Army Medical Research and Development Command (USAMRDC) received congressional authority to begin using a special type of contracting tool called an other-transaction agreement (OTA) to facilitate the delivery of advanced technology and therapeutic prototypes for a wide array of military-relevant injuries. What separates the other-transaction agreement from other standard contracting mechanisms-indeed, its defining characteristic-is that it allows for increased speed and flexibility for both parties.
Notably, it aims to set chosen projects in motion in months as opposed to the generally yearlong time span of standard agreements. That's made possible by the other-transaction mechanism's reliance on typically nontraditional defense contractors and a built-in flexibility in data rights and regulatory terms. Additionally, other-transactions allow for more conversation between the military and the performer as compared with traditional Federal Acquisition Regulation-based contracts.
"It's been exciting to watch the use of OTAs grow and expand at USAMRDC over the past few years," said Sara Langdon, the command's program manager (PM) for other-transactions with the Medical Technology Enterprise Consortium. "The flexibility has allowed for awards that involve complex collaborations with multiple companies and government laboratories, in addition to several acquisition programs."
"It is critical for us to maintain solid industry partners," said Dawn Rosarius, USAMRDC's principal assistant for acquisition. "Our labs and academic partners conduct the early research of candidate technologies to reduce risk. With less risk, our PMs can then gain commercial industry partners to not only develop the capability we need for the warfighter, but also fund some of the development, sponsor FDA [U.S. Food and Drug Administration] approval, and ensure a commercial market to lower our costs once FDA-approved."
Within USAMRDC, other-transaction efforts focused specifically on prototypes are currently awarded through the aforementioned Medical Technology Enterprise Consortium (MTEC) program, which is a 501(c)(3) nonprofit corporation consisting of industry and academic organizations committed to developing medical tools that better manage, treat and rehabilitate those suffering from traumatic injuries on the battlefield. MTEC is organized and operated through Advanced Technology International via an award obtained through competitive solicitation in 2015. Since MTEC became fully operational in January 2016, USAMRDC has awarded 42 different prototyping projects through the consortium with $131 million in government funding, with private cost-sharing efforts kicking in an additional $24 million as of August 2019.
DEVELOPING NOVEL SOLUTIONS
Leigh Anne Alexander can testify to the behind-the-scenes impact of other-transaction agreements. As deputy project manager for the USAMRDC's Warfighter Expeditionary Medicine and Treatment Project Management Office, she has used the mechanism to try to help fill a high-priority capability gap via the continued development of the Fast Onset Abdominal Management (FOAM) hemorrhage control device.
"We are trying to develop solutions that are not commercially available and very novel-solutions that industry wouldn't otherwise invest in on their own," said Alexander. "And so the ability to preconfigure the contracting agreement with options benefiting both sides was key."
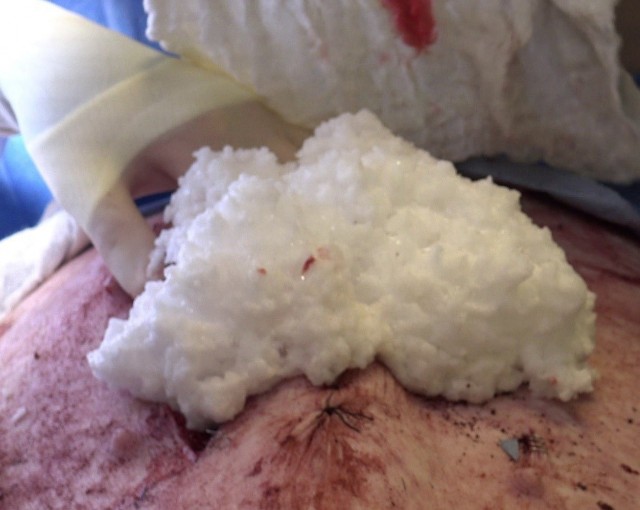
On the battlefield, certain types of injuries (and the eventual application of care to those areas) are naturally more complicated than others. While traumatic injuries to an arm or leg can be diagnosed relatively quickly and the resulting bleeding stanched with a tourniquet, traumatic injuries to the abdominal region-incidents of what is called noncompressible hemorrhage-require far more intricate care. As such, the FOAM device is designed to control severe intra-abdominal bleeding by delivering, into a Soldier's abdominal cavity, a foaming agent that eventually expands and exerts pressure on internal organs and tissue, thereby stopping internal bleeding until the Soldier can be transported to a higher level of care. At that point, the injected compound can simply be washed away by the attending surgeon.
The company behind the FOAM tool, Critical Innovations LLC was able to begin work on prototype delivery in just three to four months, using $600,000 in base other-transaction funding through the consortium. Further, the FDA granted the product its highly sought "breakthrough device" designation in an announcement in June. Alexander said the company is a full year ahead of schedule in terms of product development.
"The approach that Critical Innovations took was fantastic because they were working with the end user in mind," she said. "In the combat environment, you've got to think about the 18-year-old on the battlefield who's been through 16 weeks of training and trying to save his buddy's life."
Alexander added, "And that's because, as an Army instructor of mine once said, 'Preservation of life is at the forefront of battle.' And so in order to win the future fight, we will have to treat Soldiers farther forward on the battlefield."
Processing an effort via an other-transaction agreement does come with a cost, however. For other-transaction agreement projects routed through MTEC-and other consortia, as well-the administrative costs incorporate the additional fee as part of general operating costs.
Regardless, the perks for using other-transaction agreements are plentiful for product developers. Broad insight into all research conducted under the MTEC umbrella allows for collaboration on projects that would otherwise be conducted in silos. In addition, organizations that deliver positive outcomes may be eligible to receive additional funding for work toward FDA approval, manufacturing and procurement without needing to repeat the proposal stage of the cycle.
CONCLUSION
Back at Humacyte, other-transaction agreements issued through the consortium ultimately allowed the company to climb a slew of developmental steps in a relatively short period of time-with the warfighter the ultimate beneficiary of that boost in systemwide speed and agility. In April, for instance, the company received the MTEC Large Project Prototype of the Year award recognizing the development of the human acellular vessel, while just one month later, military surgeons in Bethesda, Maryland, performed the military health system's first-ever transplantation of one on an Army veteran in danger of losing his leg from vascular disease.
"OTAs have played a key role not only in our manufacturing and development, but also in our trauma research and clinical studies capabilities," said Blum.
From the USAMRDC's viewpoint, that kind of framework could be the path to a new, more graduated plateau of warfighter care. By investing in the identification of cutting-edge medical technology and with the creation of a dedicated avenue by which resulting prototypes are realized, the USAMRDC is allowing for science to blossom at the pace needed to forge the future of military medicine.
Said Lawson, "It's very exciting to be a part of something this transformative."
For more information go to https://mrdc.amedd.army.mil/ or https://mtec-sc.org/.
RAMIN A. KHALILI is a writer with the USAMRDC Public Affairs Office. Before assuming his current role, he spent several years as the knowledge manager for the command's Combat Casualty Care Research Program. During his prior decade-plus as a broadcast journalist, he earned an Associated Press Award for his work in Phoenix before serving as chief NASA correspondent for CBS in Orlando, Florida. He holds a B.A. in communications from Penn State University.
This article intends to convey the sentiments of a private collaborator and does not intend to endorse this specific collaborator's goods or services in any way.
This article is published in the Fall 2019 issue of Army AL&T magazine.
Related Links:
Assistant Secretary of the Army (Acquisition, Logistics and Technology)


Social Sharing