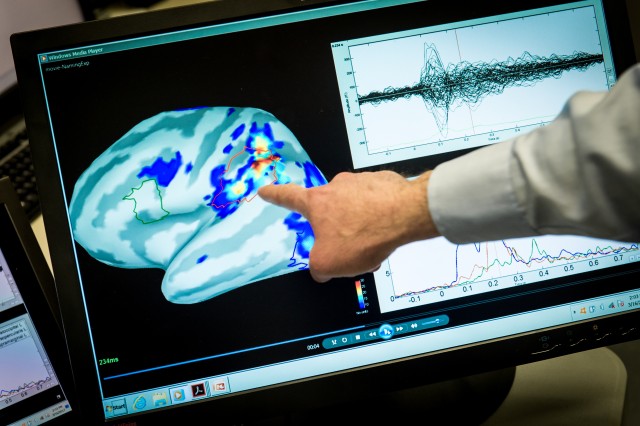
WASHINGTON -- A split second--that's all the time it takes for a brain injury to occur resulting from an accident, often changing lives forever. A percentage of these incidents may be avoided with preventative measures; however, once sustained, brain injuries can range anywhere from mild to severe.
Our Nation's Service Members face an increased risk for brain injuries due to the nature of their training and combat environments. To address this concern, the U.S. Army Medical Research and Materiel Command maintains various teams of subject matter experts and funds extramural investigators devoted to finding preventative, diagnostic and treatment methods for the wide spectrum of brain injuries.
Throughout the USAMRMC, subordinate commands and directorate organizations work in specialized medical focus areas, ranging from early medical research through U.S. Food and Drug Administration licensing and fielding, in pursuit of effective medical solutions for our Warfighters.
The Congressionally Directed Medical Research Programs - known as the CDMRP - is among the directorate organizations looking to transform health care by funding innovative and impactful medical research. As part of its mission, the CDMRP manages research awards for multiple TBI-related research efforts, from early investigative phases through translational work and forward to initial clinical trials.
The Psychological Health/Traumatic Brain Injury Research Program was established in fiscal year 2007 to address the impact of TBI on our Service Members in the Iraq and Afghanistan conflicts. In partnership with the Joint Program Committees and advanced development teams, the CDMRP provides operational execution management support as needed for the PH/TBIRP within the Defense Health Program of the Department of Defense.
"In many cases, CDMRP co-authors funding announcements with the JPC, coordinates the peer and programmatic reviews and manages the awards that result," said Dr. Dwayne Taliaferro, PH/TBIRP program manager.
"The goal of the JPCs is to translate military medical needs to military medical capabilities," he added. "This translation utilizes the knowledge and expertise of the JPCs, CDMRP, researchers and the U.S. Army Medical Materiel Development Activity."
Taliaferro further explained that when a need for additional expertise arises (e.g., product development or military technical expertise), the subject matter expertise is acquired in coordination with the JPCs. In many cases, both advanced development and CDMRP may manage awards to the same investigators or similar projects that target the same capabilities, so critical information is exchanged between the two groups to leverage each other's knowledge. In the cases where the capabilities transition to a formal acquisition program, CDMRP representatives are often included as team members to provide expertise and input.
The Combat Casualty Care Research Program, or JPC-6, is another USAMRMC directorate organization that specializes in supporting research for brain injury treatment. Its mission is to drive medical innovation through the development of knowledge and materiel solutions for acute and early management of combat-related trauma, which includes point-of-injury, en-route and facility-based care.
Dr. James B. Phillips, CCCRP's Neurotrauma portfolio manager, explained how CCCRP leads the science and technology efforts with expertise in brain trauma for early innovative solutions.
Along with laboratories throughout the USAMRMC and other DOD S&T organizations, CCCRP collaborates with an important community of expert scientists across international business and academic institutions.
"CDMRP is our execution management office that supports CCCRP with expert program-cycle management, including a robust two-tier competitive review process for brain trauma S&T research investments," said Phillips.
"Our partner at the USAMMDA has product managers who chair our integrated product teams," he said. "Through its expertise and positions for technology transition, FDA requirements [and] developmental and operational testing, USAMMDA is strategically positioned to integrate promising technology into the hands of military providers for acute and early management of brain trauma."
Phillips explained that the partnerships are important for maintaining the flow of innovative brain trauma solutions, through early and documented technology maturation and risk reduction to more advanced development for engineering and manufacturing in order to meet production and deployment as efficiently as possible.
"The rate of technology advancement can outpace previously planned solution targets, and these partnerships are necessary to stay on pace and garner the advances in technology," he added.
The CDMRP coordinates with USAMMDA to share information and includes USAMMDA representatives on programmatic panels for announcements that address military-relevant capabilities with a potential for transition to advanced development. During the review, the role of these representatives is to provide input to confirm translation potential of the proposed research in accordance with the mission of the DHP and JPC. During an award's period of performance, the CDMRP regularly provides information to the JPCs regarding the status of the research, to let them know if the project is meeting or exceeding its research objectives.
The USAMMDA serves as the USAMRMC's medical product development activity for effective solutions requested by the military. As medical gaps are identified and research matures, projects are transitioned to the USAMMDA for further development.
"The goal of this office is to rapidly develop and field FDA-approved medical solutions across the continuum of care that aid in the detection, protection, prevention and treatment of neurotrauma and psychological health conditions, such as TBI, post-traumatic stress disorder, and suicide," said Brian Dacanay, product manager for USAMMDA's Neurotrauma and Psychological Health Project Management Office.
Dacanay explained that, in order to achieve fielding of a product, the NPH PMO leads various areas that include cost, schedule, performance, concept of operations, logistics, sustainment, training, operational testing, environmental testing, risk management framework and draft capability documents.
"We are a part of the integrated product teams initiated by the JPCs," he said. "We translate research work from the JPCs/CCCRP/CDMRP into products that can be used for Service Members in pre-hospital situations."
"The earlier we initiate the partnership, the better," he continued. "This allows us to understand the research work being developed, and make changes to a device in an early stage, as necessary, that may be applicable for mass production. This synergy allows for greater cohesiveness and success as products are submitted to the FDA for approval."
As you might imagine, these are not the only partnerships required to deliver lifesaving medical solutions. Additional government partnerships involve working with industry to speed the products development process. Sometimes, the USAMRMC managed program guides the entire project, and sometimes the final approval is achieved by the company. The following five brain injury diagnostic solutions are a result of these collaborative partnerships, resulting in FDA approval:
EYE-SYNC™ (SyncThink, Inc/Brain Trauma Foundation):
This tool utilizes the process of how our eyes synchronize information to the brain. By using this tool and performing an assessment, the clinician can determine a value at which the individual's degree of vision impairment may translate to the degree of brain injury.
Ahead 100, Ahead 200, Ahead 300 devices (BrainScope):
Ahead 100 and 200 are FDA approved to be used as an adjunctive tool for the assessment of TBI. The Ahead 300 represents an evolution from the BrainScope products that have previously received FDA clearance, and, with its additional capabilities, will be the first product the company will sell commercially. The Ahead 300 features BrainScope's proprietary, patent-protected electroencephalography capabilities using sophisticated algorithms and machine learning to analyze a patient's head-injury data. Using state-of-the-art smartphone technology and a proprietary disposable electrode headset, the Ahead 300 rapidly assesses the presence of TBI in patients who present mild symptoms at the point-of-care.
Defense Automated Neurobehavioral Assessment Tool (DANA):
This tool is a mobile phone-based application designed to help medical providers identify cases of TBI in almost any setting, which can also help clinicians diagnose a patient in as little as five minutes.
Battlefield Seizure Detector for TBI Assessment (SeizTBI)/DiscoverEEG:
This device uses software algorithms as a tool to analyze electroencephalograph (from user-specified electrode number and locations) and automatically calculates conventional electroencephalograph parameters (e.g., spectral edge frequency, total power, percent alpha, asymmetry).
Banyan Biomarkers:
The Banyan Brain Trauma Indicator™ is a diagnostic blood test used to measure levels of proteins, known as UCH-L1 and GFAP, which are released from the brain into blood and measured within 12 hours of head injury. Levels of these blood proteins after a mild TBI/concussion can help predict which patients may have intracranial lesions that may be visible by a computed tomography (CT) scan and those that may not. Being able to predict if patients have a low probability of these lesions can help health care professionals manage their patients, and help to inform the decision to perform a CT scan. Test results can be available within three to four hours. This project was guided to FDA approval through USAMRMC management, with the Army Surgeon General as the Sponsor.
These devices/tools will provide medical staff with the ability to diagnose and develop treatment based on the severity of the injury. It has been proven that the moments immediately following the injury are most critical and immediate treatment can significantly improve long-term outcomes, making these tools so critical.
"Although many of the capabilities developed can apply in the civilian setting, the military faces unique environmental, mobility, interoperability, complexity and affordability challenges that must be considered," said Taliaferro. "CDRMP's role is to ensure that the DOD's needs are clearly communicated at the front end in the funding opportunity announcement and [that] the emphasis remains during the award's period of performance."
As these partnerships show, it's not merely about working hard, as much as it is about working hard together. Reaching across organizations to utilize each other's strengths is how the DOD works to advance and quickly move products out to those who need them.
Taliaferro explained how partners in industry have been able to team up with the DOD groups to continue the forward momentum of this research.
Large-scale studies like the Transforming Research and Clinical Knowledge in TBI study, National Collegiate Athletic Association-DOD Concussion Assessment, Research and Education (CARE) Consortium, Service Academy Longitudinal TBI Outcomes study, Chronic Effects of Neurotrauma Consortium, the Defense and Veterans Brain Injury Center's 15-year study, and the Warfighter Brain Health Initiative, will bridge the gap between pre-injury, concussion diagnosis and long-term outcomes. Some projects provide snapshots of what happens in the first few months of injury; however, we lack pre-injury baseline or long-term follow-up data. Similarly for research regarding individuals with chronic symptoms, we have little information on their initial injury.
"Our toolkit to diagnose TBI has improved significantly since the approval of the first blood test for TBI," said Taliaferro. "The future holds additional refinement in terms of biomarkers for diagnosis, prognosis, prediction of treatment response, and response to treatment. I think we are on the verge of an explosion of TBI advancements that will transform TBI research into TBI precision medicine."
We know preventative measures for brain injuries include wearing seatbelts in vehicles, wearing a helmet on motorcycles or bicycles and to using caution when in situations that could result in a fall. No matter how cautious we can be, it is in that split second when an accident is unavoidable that the solution discovered through medical research makes all the difference.
As long as our Service Members face brain injuries, you can be certain that these experts will continue working together to fill the research gaps towards effective medical treatments.
Social Sharing