Christopher E Hines, MD, FAPA
Chief, Outpatient Behavioral health
Scott R. Mooney, PhD, ABPP
Clinical Research Director & Board Certified Neuropsychologist
Neuroscience & Rehabilitation Center
Eisenhower Army Medical Center
FORT GORDON, Ga., (March 5, 2018) -- It is fitting that at Fort Gordon, with its emphasis on technological excellence, Eisenhower Army Medical Center is discovering new ways to use technology in the treatment of soldiers' behavioral health.
Transcranial Magnetic Stimulation, also called TMS therapy, was approved by the U.S. Food & Drug Administration in 2008 as a safe and effective medical procedure for patients with depression who have not previously benefitted from antidepressant medications.
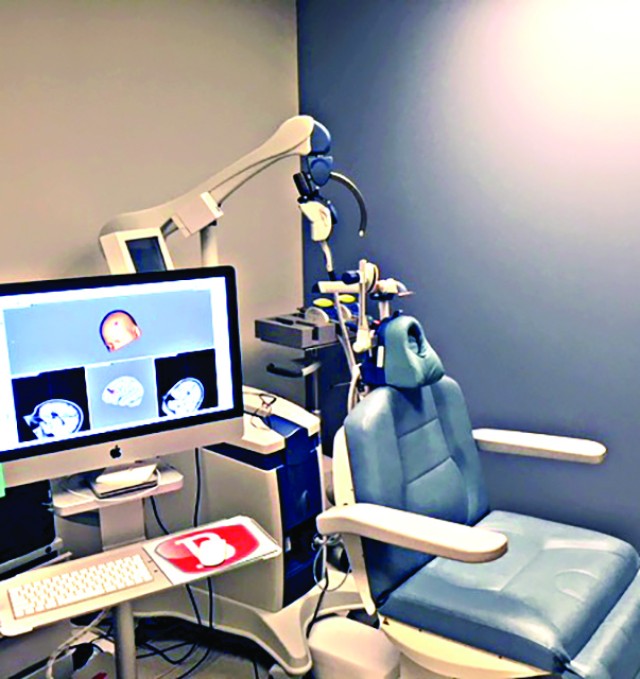
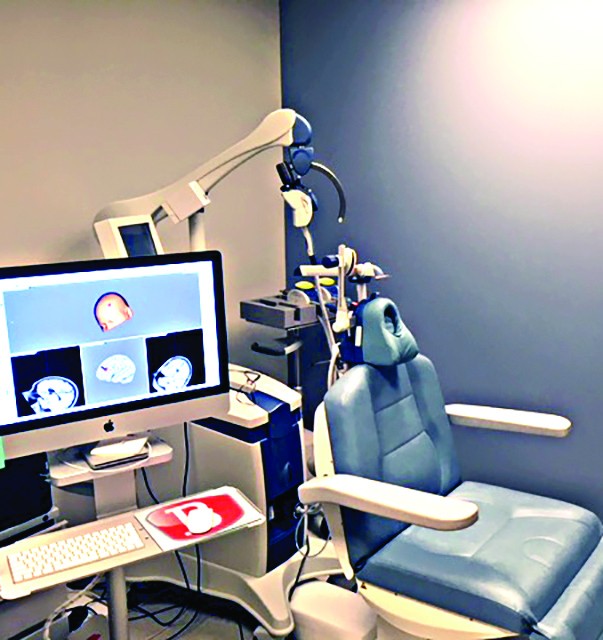
EAMC's Outpatient Behavioral Health Service is one of only four sites in the Army using TMS and has been treating soldiers since 2014. Capitalizing on Faraday's law of electromagnetic induction, TMS therapy provides electrical energy to a magnetic coil that delivers pulsed magnetic fields that are able to penetrate through the skull to selected brain regions, altering brain activity.
Depending on where the magnetic coil is placed and whether or not repetitive high- or low-frequency stimulation is used, alterations in brain activity can result in enduring changes in mood. Experientially, a doctor or member of their staff, places the magnetic coil gently on the patient's scalp in pre-defined areas.
TMS therapy is noisy with repetitive clicking noises much like an ordinary MRI machine makes. A comfortable chair and hearing protection are provided to minimize discomfort.
A typical course of TMS Therapy involves a one-hour session per day, five days a week for six weeks. Most patients who benefit from TMS therapy experience results by the fourth week of treatment. Some patients may experience results in less time, while others may take longer.
TMS is not effective for all patients with depression. Patients with a history of seizures or ferrous metal in their head that cannot be removed are generally not eligible for TMS therapy.
The most common side effect occurring while undergoing TMS therapy is headache, occurring in approximately 23 percent of patients. Seizures (sometimes called convulsions, epilepsy or fits) have also been reported with the use of TMS therapy although this is thought to be a very low risk. More than 95 percent of patients who start TMS therapy are able to finish the entire treatment course of 20-30 total sessions.
Outside of depression, promising research in TMS therapy for a myriad of psychiatric and neurological conditions is underway. At present, research studies have been conducted to determine safety and effectiveness of TMS therapy for mania, panic attacks, obsessive-compulsive disorder, schizophrenia, drug cravings, neurological conditions such as migraine and other chronic bodily pain.
EAMC-OBHS can provide TMS therapy to as many as 14 active-duty service members with depression per day with two regular TMS machines.
The TMS therapy research team uses a separate research chair that incorporates stereotactic neuro-navigation and sham (placebo-like) treatment. There are currently three research studies underway, investigating (1) if TMS therapy results in changes in sleep problems, (2) effectiveness of TMS therapy for rapid stabilization of suicidal thinking and (3) efficacy of TMS therapy for PTSD in service members with a history of concussion.
--- 30 ---
Social Sharing