FORT DETRICK, Md. -- The U.S. Army Medical Materiel Development Activity here has received a Fast Track designation from the U.S. Food and Drug Administration for the development of Tafenoquine, a potential anti-malaria drug for adults.
"Achieving FDA licensure of Tafenoquine will provide a significant improvement over the current measures of malaria prevention for U.S. forces," said Dr. Lawrence Lightner, project manager for the Pharmaceutical Systems Project Management Office at USAMMDA. "Tafenoquine is only required to be administered weekly, while current preventive measures are required daily, and it protects against all forms of malaria."
Developed through a partnership between the U.S. Army and 60 Degrees Pharmaceuticals LLC, Tafenoquine has shown promise in preventing malaria with a convenient weekly dosing regimen. Fast Track approval will allow for an expedited review of Tafenoquine's application for approval by the FDA, so that, if approved, it can be used to prevent the disease in adults traveling to malaria-prone areas.
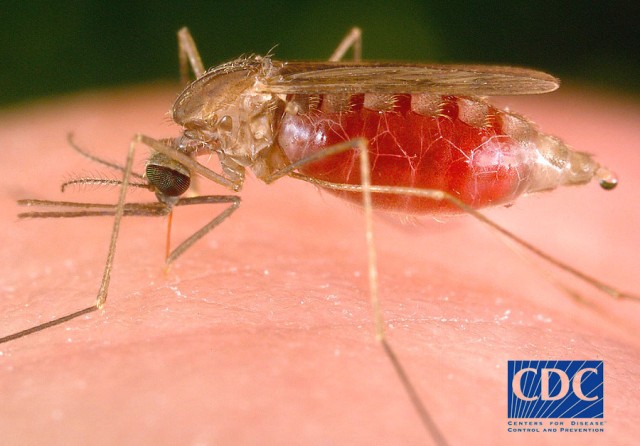
Malaria is transmitted through the bite of an infected mosquito, and remains the top infectious disease threat to U.S. military Service Members deployed overseas. The malaria parasite has several life cycle stages during infection, including a blood and liver stage, which has made treatment and prevention challenging. Tafenoquine is highly effective in the prevention of malaria, as it acts against multiple stages of the malaria parasite life cycle.
Tafenoquine was first discovered more than three decades ago at Walter Reed Army Institute of Research, and the drug eventually progressed into advanced development within the U.S. Army Medical Command. USAMMDA, a subordinate command of the U.S. Army Medical Research and Materiel Command, initiated a cooperative research and development agreement with 60 Degrees Pharmaceuticals LLC in 2014. Managed by the PSPMO, the effort has yielded successful results over the past three years in pursuit of an effective anti-malaria drug.
As the premier developer of world-class military medical capabilities, USAMMDA is responsible for developing and delivering critical products designed to protect and preserve the lives of U.S. troops across the globe. These products include drugs, vaccines, biologics, devices and medical support equipment intended to maximize survival of casualties on the battlefield.
"Our mission is to develop and deliver quality medical capabilities to protect, treat, and sustain the health of our service members," said Col. Ryan Bailey, commander of USAMMDA. "The Tafenoquine new drug application is a great example of how we intend to deliver solutions to protect our service members from the malaria threat. This achievement is the result of significant research work and product development -- a true team effort."

Social Sharing