REDSTONE ARSENAL, Ala. -- A U.S. Army Aviation and Missile Research, Development and Engineering Center senior research scientist helped develop tiny nanoparticles that convert carbon dioxide into methane using only ultraviolent light as an energy source.
Dr. Henry Everitt has been working with the chemistry department at Duke University to explore new ways light can be used to add energy to nanoscale bits of metal.
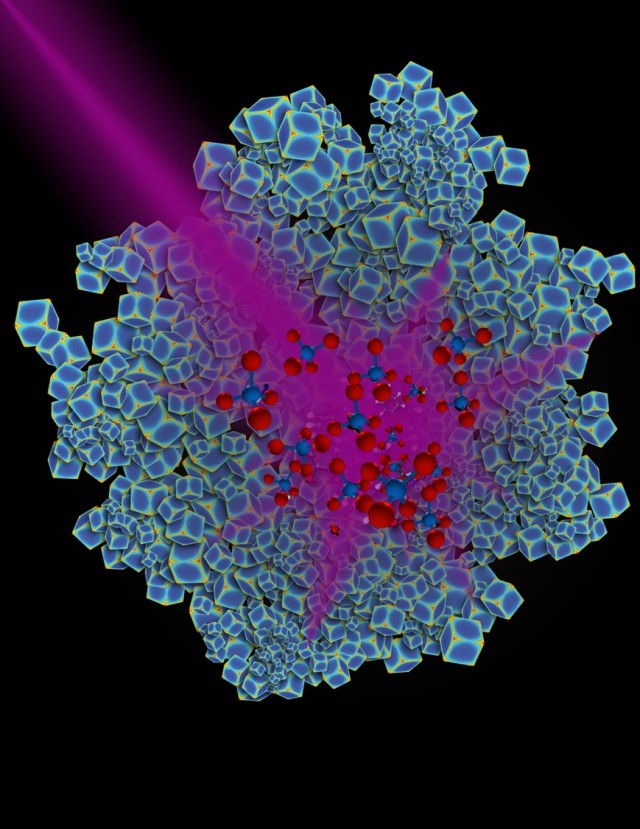
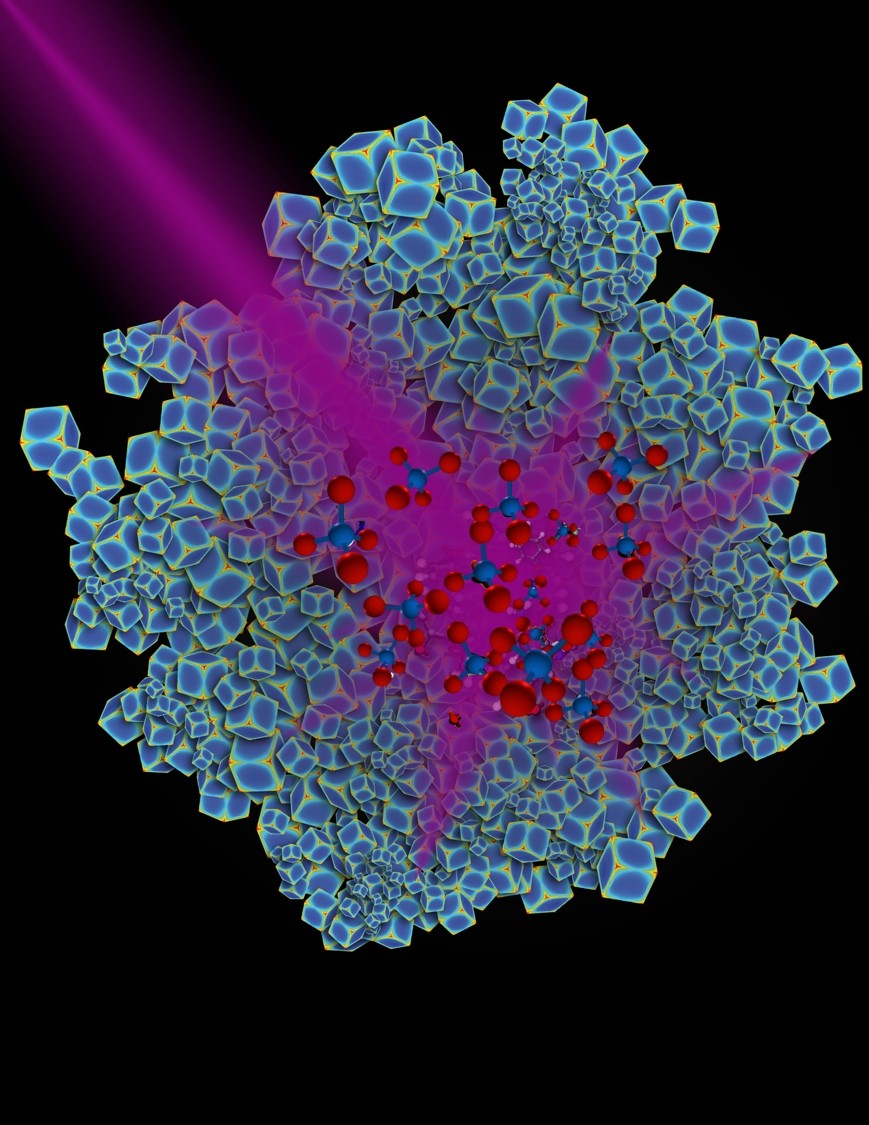
"Effectively, plasmonic metal nanoparticles act like little antennas that absorb visible or ultraviolet light very efficiently and can do a number of things like generate strong electric fields," said Everitt, who is an adjunct professor of physics at Duke University. "For the last few years there has been a recognition that this property might be applied to catalysis."
Scientists have sought an efficient, light-driven catalyst to power this light-using process, which could help the growing levels of carbon dioxide in the atmosphere by converting it to methane. Methane is a building block for many types of fuels.
By heating nanoparticles to 300 degrees Celsius, the Duke lab observed that the reaction generates an equal mix of methane and carbon monoxide, a poisonous gas. When the heat was turned off and particles were illuminated with a high-powered ultraviolent LED lamp, the carbon dioxide and hydrogen reacted at room temperature, almost exclusively producing methane.
"We discovered that when we shine light on rhodium nanostructures, we can force the chemical reaction to go in one direction more than another," Everitt said. "So we get to choose how the reaction goes with light in a way that we can't do with heat."
"The fact that you can use light to influence a specific reaction pathway is very exciting," said Dr. Jie Liu, the George B. Geller professor of chemistry at Duke University. "This discovery will really advance the understanding of catalysis. This sort of analysis can be applied to many important chemical reactions, and we have only just begun to explore this exciting new approach to catalysis."
The scientists published their research and findings in an online publication, Nature Communications, Feb. 23. Nature Communications is an open access journal that publishes high-quality research from all areas of the natural sciences. Papers published by the journal represent important advances of significance to specialists within each field.
The team plans to test whether its light-powered technique might drive other reactions that are currently catalyzed with heated rhodium metal. They hope to develop a version of the catalyst that is powered by sunlight, creating solar-powered reaction that could be integrated into renewable energy systems.
The article, "Product selectivity in plasmonic photocatalysis for carbon dioxide hydrogenation" can be found at the following link: http://www.nature.com/articles/ncomms14542
---
The U.S. Army Aviation and Missile Research, Development and Engineering Center is part of the U.S. Army Research, Development and Engineering Command, which has the mission to provide innovative research, development and engineering to produce capabilities that provide decisive overmatch to the Army against the complexities of the current and future operating environments in support of the joint warfighter and the nation. RDECOM is a major subordinate command of the U.S. Army Materiel Command.
Related Links:
Army.mil: Science and Technology News
U.S. Army Aviation and Missile Command
U.S. Army Research, Development and Engineering Command
U.S. Army Aviation and Missile Research, Development, and Engineering Center
Social Sharing