The U.S. Army Medical Materiel Development Activity could not let 2016 end without achieving one more milestone. In December, AcelRx Pharmaceuticals, Inc., a development partner of USAMMDA, submitted a New Drug Application to the U.S. Food and Drug Administration for the pain management drug, Sufentanil NanoTabTM.
The NDA was filed for use of the Sufentanil NanoTabTM for treatment of moderate-to-severe pain. This filing was the direct result of the partnership developed between AcelRx and USAMMDA and will lead to the eventual licensing and fielding of this treatment for U.S. forces.
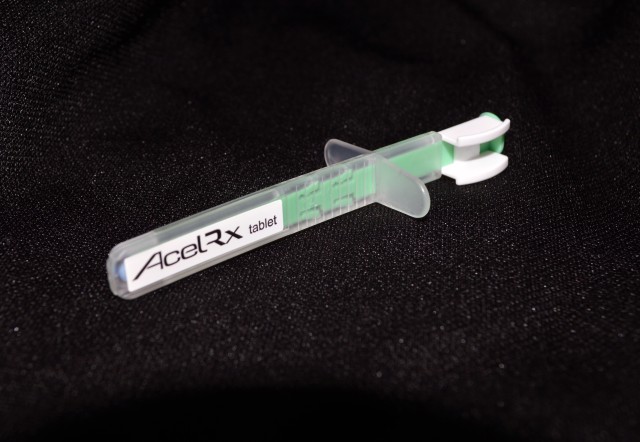
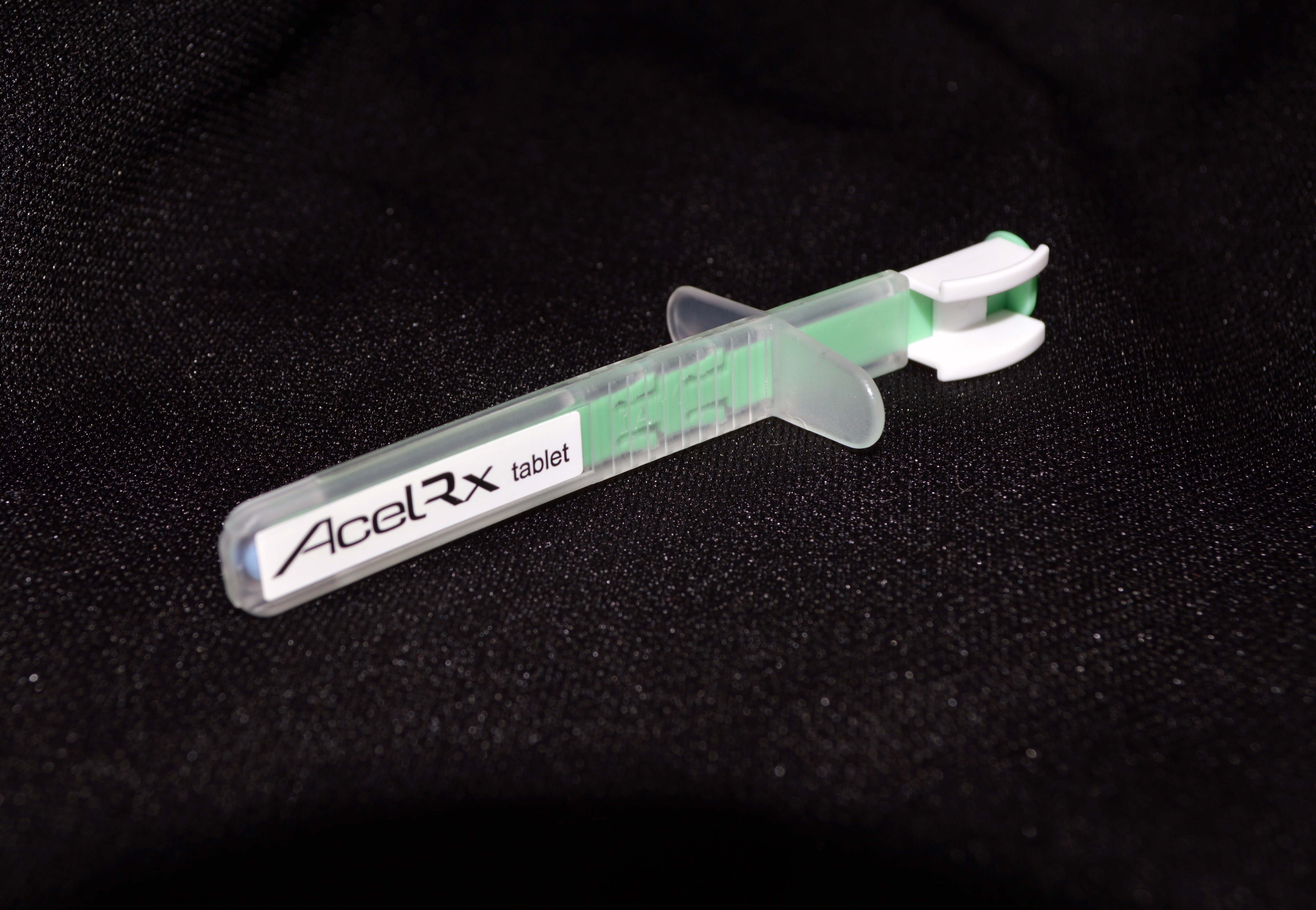
The Sufentanil NanoTabTM product consists of 30 microgram Sufentanil tablets in a pre-filled, proprietary, single-dose applicator. The medication is delivered sublingually (under the tongue) to the patient by a healthcare professional. This method of delivery is what makes the treatment so effective, especially on the battlefield where it can be most difficult to administer effective, fast-acting pain management treatment.
The sublingual method of application allows faster absorption of the medicine. Due to the many capillaries (blood vessels) found under the tongue, the medication can be absorbed directly into the bloodstream. The current standard of care for battlefield pain management is a morphine sulfate autoinjector, administered via intramuscular injection.
Andy Atkinson, product manager in USAMMDA's Pharmaceutical Systems Project Management Office, describes the current battlefield pain management options as more invasive and problematic.
"The limitations of morphine include an incomplete ability to relieve pain, as well as unfavorable side effects when used in progressively higher doses, and decreased physical and cognitive capability of the Soldier," said Atkinson. "Unfavorable side effects of this opioid drug include, but are not limited to, cardiovascular depression, respiratory depression, dependence and sedation.
"Further, the IM route of administration is problematic in situations of hemorrhage, hypovalemia (decrease in volume of blood plasma) and hypothermia, where absorption is poor, relief from pain takes longer to effect, and the risk of overdose is high with volume resuscitation during later stages of care."
Atkinson explained that after concluding four clinical trials, it appeared that patients treated with the Sufentanil NanoTabTM experienced improvements in pain levels as early as within 10 to 15 minutes. Overall, the Sufentanil NanoTabTM has proven to have a higher therapeutic and safety index than morphine injection, as well as a less invasive delivery method.
Dr. Larry Lightner, project manager for the USAMMDA PSPMO, described the USAMMDA's role as overall manager, in regard to the sufentanil development effort.
"The advanced clinical studies of the effectiveness of this product were funded, in part, with Defense Health Program Research, Development, Test and Evaluation funding from the Clinical and Rehabilitative Medicine Research Program. Because the funding was from the advanced development program, the PSPMO of USAMMDA assumed the lead for overall management of the effort, including management of the U.S. Army Medical Research and Materiel Command's contract with AcelRx," said Lightner.
"This included not only working with AcelRx," he continued, "but also working closely with the Army Medical Department Center and School to ensure that the appropriate requirements documents were in place and all training issues were resolved. Additionally, the PSPMO was responsible for ensuring that all USAMRMC human use and acquisition requirements were met during the development process."
The filing of a NDA is a major accomplishment, since this is basically the last step before FDA licensing. The final step is the FDA auditing process, to address any potential issues. Atkinson explained how AcelRx will also be preparing for an FDA-sponsored advisory committee during the review period.
"This is a result of a recently enacted FDA Opioid Action Plan which requires the FDA to convene an expert independent advisory committee prior to NDA approval of any new opioid drug [the only exception is for new opioid drugs that contain abuse-deterrent properties]."
Col. William E. Geesey, USAMMDA commander, offered his thoughts on the significance of this achievement and the dedication of the entire organization.
"This is great work that takes the effort of the whole team," said Geesey. "These are significant milestones that we recognize too infrequently due to the nature of our business -- so our group should be very proud of what we've accomplished."
As 2016 has come to a close, one thing remains clear: the Warfighters' health and safety are at the forefront of USAMMDA's efforts. The USAMMDA mission to develop and deliver quality medical capabilities to protect, treat and sustain the health of Service Members throughout the world is evidenced by this great achievement.
FDA licensing for the Sufentanil NanoTabTM is anticipated by the end of 2017.


Social Sharing