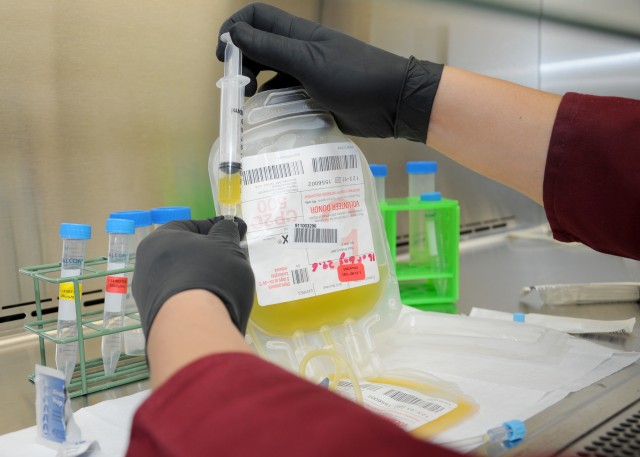
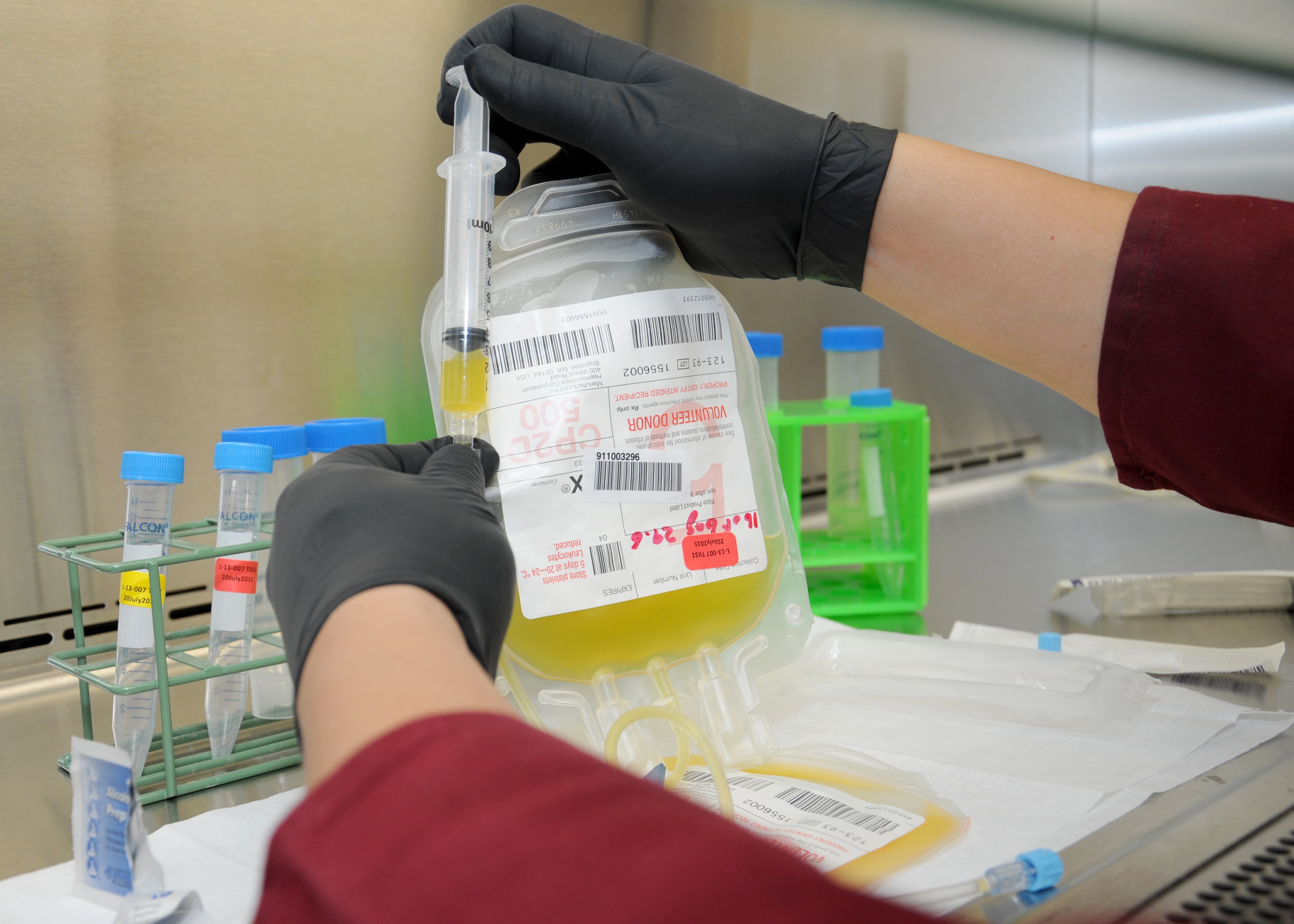
On June 29, the U.S. Food and Drug Administration announced the approval of cold-stored apheresis platelets for the resuscitation of bleeding patients. Apheresis platelets are removed from a donor's blood that has been passed through a device that separates the platelets and returns the blood back to the donor.
The agreement by the FDA allows storage of apheresis platelets for three days at refrigerator temperature, which is between 1 to 6 degrees Celsius or 33.8 to 42.8 degrees Fahrenheit.
The change is welcomed by military researchers at the U.S. Army Institute of Surgical Research at Fort Sam Houston, Texas, who see this as a first step to the further development of cold-stored platelets for treatment of combat Wounded Warriors on the battlefield.
Platelets are a vital component in blood that combines with red blood cells and plasma to form clots that stop or minimize blood loss. Trauma patients with severe bleeding can be transfused with platelets to assist with coagulation.
"This was a very important decision from the FDA," said Heather Pidcoke, Ph.D., research physiologist and deputy task area manager of the Coagulation and Blood Research Program at the USAISR.
According to Pidcoke, research conducted at the USAISR on platelets collected by apheresis and stored in the cold shows that the platelets retain blood clotting qualities longer and are less likely to be contaminated by bacteria than the current standard-of-care room temperature stored platelets.
"We found that platelets were active and functional in the cold up to 14 days, whereas we found much diminished aggregation response in the platelets stored at room temperature after day three," said Pidcoke.
"We will now be able to conduct research on extending the FDA-approved storage time of cold-stored apheresis platelets to beyond three days," said Lt. Col. (Dr.) Andrew Cap, chief of the USAISR Coagulation and Blood Research Program.
"That's where our research is focusing on now," Pidcoke added.
Before the change by the FDA, bleeding patients could only be transfused with apheresis platelets stored at room temperature, which circulate in the body for longer periods of time than cold-stored platelets, but lose most of their blood clotting function during storage. According to the FDA regulations platelets can be stored at room temperature for only five days because of the increased risk of bacterial growth in room temperature products. This risk forces hospitals to quarantine platelets for bacterial testing before transfusion.
"That takes two days," Pidcoke said. "If nothing is growing on day two, then it can be transported or used on or after day three when the results are known. In essence, room temperature platelets only have a three day functional shelf life -- like cold-stored platelets."
Cold storage of apheresis platelets will make them safer and will allow them to be used immediately without bacterial testing, like red blood cells. Pidcoke added that there are other benefits to cold storage of apheresis platelets.
"Cold-stored platelet bags can be added to red blood cell and plasma bags in a chilled 'golden hour' blood transport box," Pidcoke said. "This makes it practical to give platelets on the battlefield."
Another benefit of cold-stored platelets is that the need for a machine to shake the room temperature stored platelets is eliminated which reduces the footprint or equipment needed in a battlefield operating room.
"If you don't have platelets for trauma patients, you are going to have a hard time saving lives," said Pidcoke. "The research here at ISR has taken a major step forward in trying to achieve that goal."
Social Sharing